Incorrect labelling error forces Endo US Inc. to recall Clonazepam
The recall is due to its life-threatening impact on human life
In what has been described as a labeling error by the third-party packager, Endo, Inc., the manufacturer of Clonazepam recalled the anxiety drug, according to a notice shared by the FDA on Nov. 19. The recall was earlier announced on July 16th, this year.
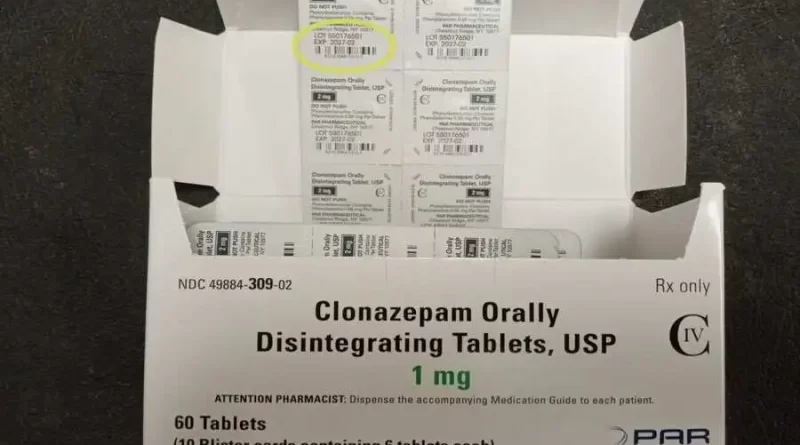
The orally-disintegrating tablets due to human error have been called back, which are possibly life-threatening. The reason is that certain lots have been printed with an incorrect strength and National Drug Code (NDC) code. Surprisingly, the name of the previous company, Par Pharmaceutical, which used to market Clonazepam before Endo bought it, is also mentioned on the package.

Incorrectly labeled Clonazepam packs a threat to health
Consumed by a large number of masses in the US, inadvertent use of Clonazepam, which is also the generic name for Klonopin, carried with it risks like sedation, confusion, dizziness, diminished reflexes, ataxia, and hypotonia,” said the company in a notice on the US Food and Drug Administration (FDA) website. The recalled product comes in cartons containing 60 tablets packed into 10 blister strips each containing six tablets.
Endo Inc., the company based in Pennsylvania, also added that “There is a reasonable probability for significant, possibly life-threatening, respiratory depression especially for patients with concomitant pulmonary disease, patients who have prescribed dosing near maximal dosing, and patients also taking other medications that could cause additional respiratory depression.”
This sort of depression forces people to breathe too slowly, which ultimately leads to a low amount of oxygen level in the blood, which is considered very dangerous for the patient, as reported by Cleaveland Clinic.
While consumers have been advised to stop consuming the tablets, a physician needs to be contacted in case of accidental usage. A possibility of adverse reactions as described above has been indicated in the case of children and adults.
The Company also made arrangements through Inmar, Inc., to ensure all the inventory returns are made. Again, Endo provided written notifications to all its retailers and wholesalers to stop distributing the drug at once in every place.
Treatment through Clonazepam
While the strips inside the boxes carry the proper strength, the error has been noticed on the cartons containing the tablet strips. The carton and each blister strip pocket are printed with the name, strength, lot number, expiration date, and NDC number.
Panic, anxiety and seizures are treated with Clonazepam (USP, C-IV). The drug has a certain mechanism by which it works and involves a marked decrease in abnormality of the electrical brain activity. The U.S. National Library of Medicine further says that Clonazepam comes in a class of medications called benzodiazepines.
Taken usually before or after meals, the drug controls certain kinds of seizures and panic attacks as per the NLM. Before Clonazepam was developed further by Endo, the packs had “Distributed by: Par Pharmaceutical,” printed on them. Endo Inc. had brought into effect the recall earlier and is now bringing to notice the said error, to the wholesale accounts and retailers, trying to get the packs returned via Inmar Inc. And that is supposed to be on a nationwide scale.